About Rehabilomics® Research
The University of Pittsburgh Department of Physical Medicine & Rehabilitation (PM&R) is on the leading edge of TBI research and actively working to translate research outcomes to effective treatments for people with TBI with the goal of improving the lives of all who are affected.
Model System Research
Dopamine Dysfunction in TBI: A contexturaliezed Rehabilomics® Investigation Using an ICF Framework for Assessing Functioning, Disability, and Health
Principal Investigator: Amy Wagner, MD
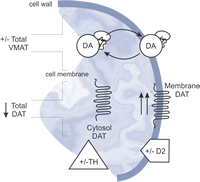
Traumatic brain injury (TBI) is known to cause significant complications, including seizures, hypogonadism and depression. Unfortunately, it is very difficult to predict who will experience these complications because people with similar trauma and comparable hospital and rehabilitative care often display very different outcomes. However, recent research by our lab has shown that genetic variants can serve as strong predictors of both outcome and complications after TBI. Specifically, evidence suggests that dopamine (DA) dysfunction underlies many of the cognitive deficits observed after TBI and that DA enhancing drugs can improve cognition and recovery. This research project will further refine our understanding of DA dysfunction and provide an infrastructure and exemplar from which future studies can build upon to effectively reduce DA dysfunction and to optimize recovery through personalized treatment of persons with TBI.
Pilot Feasibility Study: Ecological Momentary Assessment (EMA) Tools for Rehabilitation
definition: ecological momentary assessment (EMA) refers to a method by which a research participant reports on symptoms, behavior, task completion, etc in "real time" or close to "real time" in experience.
Principal Investigator: Amy Wagner,MD
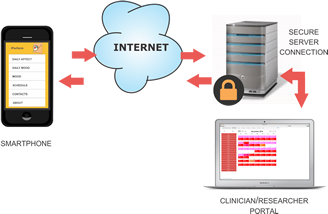
We have developed, with the aid of RERC on Telerehabilitation (NIDRR funded project H133E090002), relevant mobile phone apps to test the feasibility of EMA symptom journaling with individuals with TBI and their caregivers.
Phase 1 will require participants to trial mobile health tools to track TBI-specific symptoms, complications, and outcomes across TBI recovery and to provide feedback on usability. The development team will use the feedback to address usability problems and optimize the design of each app.
In Phase 2 of the pilot study, patients and caregivers will receive orientation to the system and then be asked to perform tasks for each of the apps (e.g., setting up a medication schedule, responding to reminders, answering mental health questionnaires, writing personal journals for tracking symptoms).
Data accuracy and completeness, as well as participant satisfaction with the system, will be assessed.
The ability to characterize and track people prospectively with a high level of temporal resolution (e.g., symptom tracking in “real time”) provides a rich landscape upon which to generate important questions focused on longitudinal outcomes. Importantly, this technology provides a mechanism for patients to report problems/symptoms to a coordinator, and for the coordinator to provide alerts/reminders to the patient. This 2-way data exchange protocol is innovative and unlike any product on the market, and it addresses major weaknesses of already existing apps and mHealth systems for chronic conditions.

